Hello everyone! Since this is my first post, I figured I would introduce myself a bit and then get right to the science. My name is Skyler and I’m a rising junior from Washington state. I’m a biology major with a neuroscience emphasis and am considering a minor in chemistry (we’ll see about that one). I’m also part of the Honors Program. I’ll be going abroad to Denmark next semester (and I’ll have a study abroad blog too!), but before that happens I’ll be doing research here on campus for the summer.
My research is neuroscience based but also includes some ecology, cell biology, and a little chemistry. I am studying the effects of bisphenol A, a chemical found in plastics, on the development and reproduction of aquatic snails Helisoma and Lymnaea. BPA can leach out of plastic in landfills and get into waterways, causing a host of reproductive issues for the species that are exposed. Its chemical structure has similar properties to the binding site of estrogen, so it is able to mimic estrogen in the brain and cause unnecessary hormone release. Regulations of this chemical are not very strict, as its effects on aquatic wildlife are largely unknown. Pulmonates (aquatic snails that can also live on land) are good indicator species for this type of aquatic pollution because they’re easy to breed and keep in a lab setting, have relatively short life cycles, and produce lots and lots of egg masses. Snail hormone systems are also comparable to human hormones.

Lots of Helisoma snails hanging out on their second favorite thing — lettuce (their first favorite is watermelon juice).
So far I’ve been experimenting with finding the best habitat conditions in which to keep the eggs. The egg masses are laid in the main tanks where the adult snails are kept, but I remove them and have been putting them in small glass petri dishes. The egg masses are kept incubated in water usually used for breeding fish (aquarium salt water with a little bit of chemicals added), but when they hatch I move the babies to dishes either containing fish water or BPA water. Our starting concentration of BPA is 200 micrograms per liter, but in the future we’ll play around with higher and lower concentrations. The water was being aerated in its bottle but not directly in the dish because the dishes were too small. Generally I placed two to five snails in each dish and observed them daily. This arrangement didn’t seem to suit the snails, though, and most of them have died in this setup. I’ve now switched to using the plastic boxes that micropipette tips come in, which are large enough that I can aerate the water directly. The hatchlings seem to like it better in there, by which I mean none of them have died so far. Their diet consists of a drop of watermelon juice per snail daily, but those still in the dishes with egg masses also like to munch on the empty egg sacs.
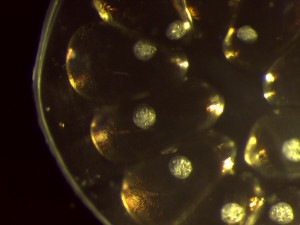
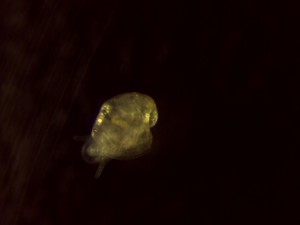
For the most part I’ve been doing daily observations of the egg masses and hatchlings using a microscope that hooks up directly to my computer via USB. This allows me to take pictures and short video clips of them to better document their growth. The pictures below were taken with the microscope and show the different stages of growth in and outside of the eggs. Next time I’ll talk about dissection and post some pictures of snail brains!